Age of the Earth
The age of the Earth as determined by the current method of radiometric dating is about 4600 million years. Before the discovery of radiometric dating, several attempts were made to determine the age of the Earth. The important ones are as follows.
- From the discovery of organic evolution
- From the rate of sedimentation
- From the salinity of seawater
- From the rate of cooling
From the history of organic evolution
As soon as the Earth cooled and solidified, life appeared on it. Initially, the organism had a simple body structure. The complexity increased with the passage of time. Man who has evolved quite recently, possesses the most complex structure. From the history of organic evolution, biologists have determined the age of the Earth to be about 1000 million years.
From the rate of sedimentation
The formation of sedimentary rocks started with the solidification of the Earth. Hence efforts were made to calculate the age of the Earth from the rate of sedimentation in the ocean. The method involved was to determine the total thickness of sedimentary rocks that had been deposited during the Earth’s history and then divide it by the rate of sedimentation. This method gave an age of around 500 million years. This method had many difficulties, some of which are as follows.
- Different sediments accumulate at different rates under varying conditions.
- A rate of sedimentation determined for recent sediments can not necessarily be applied to the past.
From the salinity of seawater
It is assumed that the oceans originally had fresh water. If the amount of salt carried to the ocean each year by rivers is known, it can be calculated how much time it took to accumulate the salt now present in the ocean. This method gave an age of about 120 million years. This estimate was not accurate as the rate of accumulation of salts could never have been uniform all through the history of Earth.
From the rate of cooling
In 1897, the British physical Lord Kelvin calculated the age of the Earth from the temperature difference between the initial molten planet and its present state. He assumed that the rate of heat loss was constant throughout the Earth’s history. This method gave an age of 20 to 40 million years, which was more than 100 times lower than current estimates.
Kelvin also made estimates based on assumptions concerning the origin of the sun’s heat. The age of the earth calculated by this method gave similar results that is the figures ranged from 20 to 40 million years. Kelvin’s estimate for the sun and the earth were wrong because he did not take into account the fierce heat generated by the decay of radioactive elements.
Radiometric dating
The methods of radiometric dating are based on the radioactive decay of certain isotopes that have very long half-lives. The radioactive isotope is often referred to as the “parent” and the elements resulting from the decay of the parent are called the “daughter products”. The age of rocks and minerals that contain radioactive isotopes is determined by measuring the accumulation of the daughter products in them. This procedure is called “radiometric dating”. Radiometric dating is very reliable because the radioactive decay proceeds at a constant rate and it remains unaffected by any physical or chemical agents.
A commonly used term in radiometric dating is “half-life. It is a common way of expressing the rate of radioactive decay. Half-life may be defined as the time required for a given amount of radioactive substance to decay to one-half of its initial value. For example, the half-life period of uranium-238 is 4500 million years. This means that in 4500 million years one gram of U-238 would be reduced to a half gram, the rest having been transformed into the Lead-206.
Of the many radioactive isotopes that exist in nature, only three have proved useful in providing radiometric ages for ancient rocks. These three isotopes are given in the following table. The methods which is commonly used for dating rocks include Potassium-Argon, Rubidium-Strontium, and Uranium-Lead.
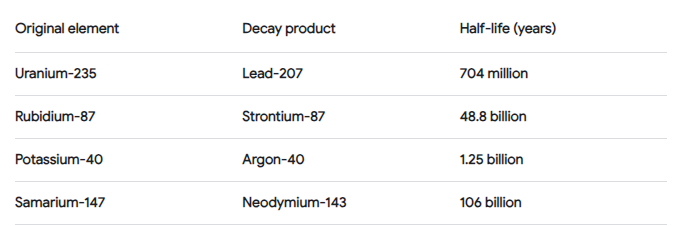
The basic equation for calculating the age of a rock sample is as follows.
Age of rock= 3.323 T log 10 {1+Nd/Np}
where Nd is the number of atoms of the daughter product, Np is the number of atoms of the parent radioactive substance present today and T is the half-life of the radioactive substance.
In practice, mass spectrometers are used to measure precisely and accurately the quantities of parent and daughter radioactive substances in rocks. In radioactive dating, it is assumed that none of the daughter product has been leached out from the rock and that none has been introduced from outside.
The radiometric dating method gives a figure of some 3500 million years for the oldest rocks of the Earth’s crust. The radioactive analysis of meteorites has yielded dates of up to 4600 million years, which probably correspond to the age of the earth.
You may like:



Leave a Reply