What is radioactive decay?
Radioactive elements continuously release their protons and neutrons and form new daughter products. The radioactive elements are often known as the “parent”, and the element resulting from the decay is known as the “daughter product”. As the number of protons and neutrons is different in parent and daughter products, therefore the daughter product becomes a quite different element. This process is known as radioactive decay, and the time taken for the conversion of half of the parent element into the daughter product is known as the “half-life”. Common Radiometric Methods
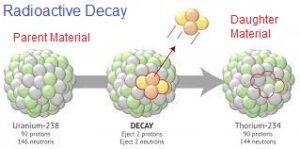
Science enabled us to know the half-lives of the radioactive isotopes found on Earth, and so we can trace, for how long a radioactive material within an object has been decaying by measuring its decayed quantity (daughter product).
Several radioactive materials decay into daughter products that are also radioactive, and have their half-life: the result is called a “decay-chain”, which eventually decays into a non-radioactive substance.
Radiometric Dating
Radiometric dating is used to determine the age of the rocks. The methods of radiometric dating are based on the radioactive decay of a particular radioactive isotope. A commonly used term in radiometric dating is “half-life”. It is a common way of expressing the rate of radioactive decay. Half-life may be defined as the time required for a given amount of radioactive substance to decay to one-half of its initial value. For example, the half-life period of Uranium-238 isotope is 4.5 billion (4500 million) years. This means that in 4500 million years one gram of Uranium would be reduced to a half gram, the rest having been transformed into the lead-206. Like Uranium, most of the isotopes have a very long half-life. The age of the rock and minerals that contain radioactive isotopes is determined by measuring the accumulation of daughter products in them. This procedure is called radiometric dating. In this method of dating, we divide the half-life of a radioactive element by its decayed portion. Let’s suppose, we find an amount of Uranium in a rock, with 20 % (5th part) part of it decayed. We know the half-life of Uranium, that is 4.5 billion years.
Age of the Rock= 4500000000 /5 = 900000000 years
where 4500000000 is the half-life of the rock, 5 is the 1/5th part of the total amount of Uranium, and 900000000 is the exact age of the rock.
Radiometric dating is very reliable because the radioactive decay proceeds at a constant rate and it remains unaffected by any physical and chemical agents.
Common Radiometric Methods
1. Potassium-Argon Method
Potassium-40 has a half-life of 11900 million years. It is commonly found in many rocks. it decays into argon-40, an inert gas found in the earth’s atmosphere, hydrosphere, and biosphere. The Potassium-Argon method can be used to date rocks that are millions of years old, including (age 4600 million years). However, rocks younger than 0.1 million years are difficult to date because they contain very little radiogenic argon. The minerals that are suitable for K-Ar dating include biotite, muscovite, hornblende, and nepheline. the potassium felspars, such as orthoclase and microcline are unsuitable for K-Ar dating because they are likely to lose argon readily at atmospheric temperatures.
2. Rubidium-Strontium Method
Rubidium-87 has a half-life of 50,000 million years. The Rb-Sr method can be applied to rocks of almost any geological age. However, rocks younger than 20 to 30 million years may create analytical difficulties because of the minute amount of radiogenic Sr-87 present. The Rb-Sr method can be used to date such common rock-forming minerals as muscovite, biotite, and all types of potassium felspars including orth0-clase and microcline.
3. Uranium-Lead Method
The half-life of Uranium-238 is 4500 million years. Uranium and thorium frequently occur in the same mineral and it is therefore possible to make two independent age determinations on one mineral sample. The U-Pb method can be applied over the greater part of the geological age range, although their usefulness decreases sharply for rocks younger than 100-200 million years. This method is less commonly used than the K-Ar and Rb-Sr method but may be used to date rarely minerals in which uranium and thorium are major constituents. It is frequently applied to minerals such as zircon and sphene.
4. Radiocarbon Dating
There are three isotypes of carbon: C-12, C-13, and C-14. Of these C-14 is a radioactive element. Since it has a half-life of only 5730 years, it can be used to date events in recent geological history. Although C-14 is only useful in dating the last small fraction of the geological time scale (75,000 years), it has become an important means for dating ice age and archaeological material.
Radiocarbon (C-14) is produced in the atmosphere by cosmic ray bombardment of nitrogen-14. The C-14 combines with oxygen to form carbon dioxide, which is absorbed by living organisms. A constant ratio of C-14 to C-12 is established in each organism during its life. After death no more carbon dioxide is absorbed and the C-14 decays steadily by emitting beta particles to N-14.
You may Like to read:

Leave a Reply